An Update on the ACIP 2019-2020 Influenza Vaccine Recommendations - Pharmacy Times
Kimberly C. McKeirnan, PharmD, BCACP
2018-2019 ACIP INFLUENZA SURVEILLANCE
At the ACIP meeting in February 2019, a surveillance update was provided with statistics regarding the 2018-2019 flu season. According to the CDC, between October 1, 2018, and February 16, 2019, there were between 17.7 million and 20.4 million flu illnesses, between 8.2 million and 9.6 million flu-related medical visits, between 214,000 and 256,000 flu-related hospitalizations, and between 13,600 and 22,300 flu-related deaths.3 Additional data presented at that meeting revealed that during the 2017-2018 flu season, influenza vaccination prevented 7.1 million cases of the flu, 109,000 hospitalizations, and 8000 deaths.4 The interim results of the 2018-2019 flu season (through February 2, 2019) are summarized in table 1.5

Many pharmacy patients are skeptical about the efficacy of seasonal flu vaccine. However, these statistics support the ongoing need for pharmacists to emphasize the importance of an annual influenza vaccine to their patients.
AN UPDATE ON THE ACIP RECOMMENDATIONS RELATED TO INFLUENZA VACCINES
Recommendations provided by the ACIP about the use of each vaccine are developed after the committee critically reviews a great deal of vaccine-related information, including disease epidemiology and burden, vaccine efficacy and effectiveness, vaccine safety, quality of evidence, feasibility of program implementation, and economic analyses of immunization policy.6 Every year, the child and adult immunization schedules are published with updated vaccination recommendations.7,8 These documents are meant to help health care providers select the best vaccine and appropriate timing for each patient.
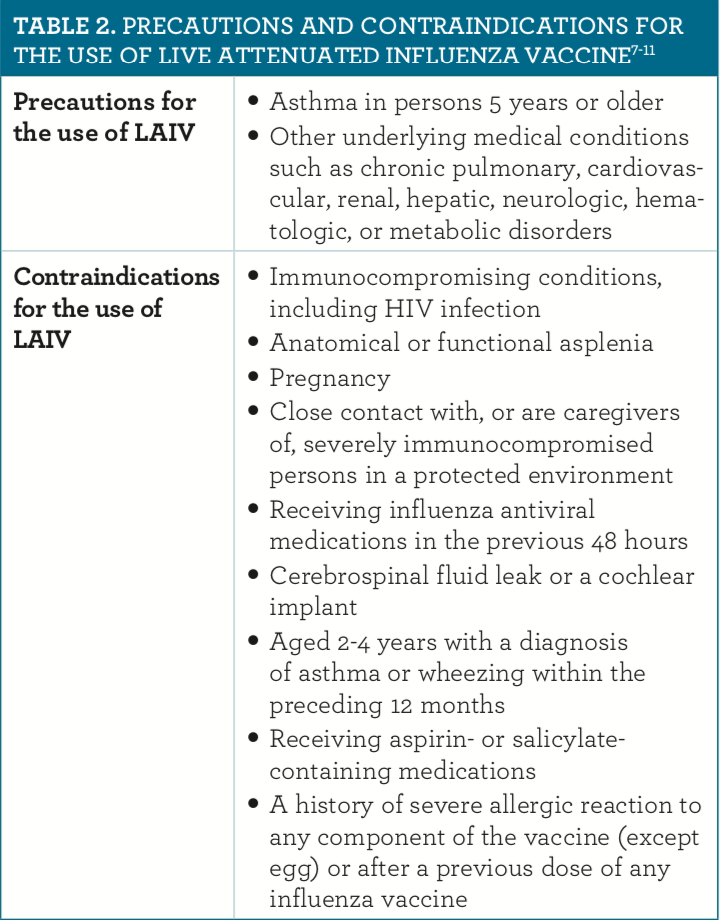
In the 2019 updates to the immunization schedules, a notable change was the addition of the live attenuated influenza vaccine (LAIV).9-11 The immunization schedule for all individuals between the ages of 2 and 49 years includes the LAIV unless 1 of the contraindications or precautions listed in table 2 is present.7-11 The ACIP also added a recommendation that any licensed influenza vaccine that is appropriate for the age and health status of a patient may be used.11 Although the LAIV was added to the list of approved vaccines, no specific preference was given to the use of either the LAIV or the inactivated influenza vaccine.8 The ACIP recommendations also include a Special Situations section that provides guidance on vaccinating patients who report an allergy to eggs.7-11 These recommendations are summarized in table 3.7-11

CONCLUSION
The February 2019 ACIP meeting led to important changes in recommendations for influenza vaccine administration. Since these recommendations change frequently, it is a best practice for pharmacists to stay up-to-date on the most recent vaccination schedules and ACIP recommendations.
Kimberly C. McKeirnan, PharmD, BCACP, is a clinical assistant professor in the department of pharmacotherapy at the Washington State University College of Pharmacy and Pharmaceutical Sciences in Spokane, Washington.
REFERENCES
- Advisory Committee on Immunization Practices (ACIP): general committee-related information. CDC website. cdc.gov/vaccines/acip/committee/index.html. Reviewed October 23, 2018. Accessed May 14, 2019.
- Advisory Committee on Immunization Practices (ACIP). ACIP recommendations. CDC website. cdc.gov/vaccines/acip/recommendations.html. Reviewed March 21, 2019. Accessed May 14, 2019.
- Brammer L. National Center for Immunization & Respiratory Diseases Advisory Committee on Immunization Practices: influenza surveillance update. CDC website. cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/flu-2-Brammer-508.pdf. Published February 27, 2019. Accessed May 14, 2019.
- Rolfes M, Flannery B, Chung J, et al; U.S. Flu VE Network; the Influenza Hospitalization Surveillance Network (FluSurv-NET); Assessment Branch, Immunization Services Division, Centers for Disease Control and Prevention. Effects of influenza vaccination in the United States during the 2017-2018 influenza season [published online February 2, 2019]. Clin Infect Dis. doi: 10.1093/cid/ciz075.
- Flannery B, Chung J, Doyle J. National Center for Immunization & Respiratory Diseases: interim estimates of 2018-19 seasonal influenza vaccine effectiveness against medically attended influenza from the US Flu VE Network. CDC website. cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/flu-3-Flannery-508. pdf. Published February 27, 2019. Accessed May 14, 2019.
- US Department of Health and Human Services. Charter of the Advisory Committee on Immunization Practices. CDC website. cdc.gov/vaccines/acip/committee/acip-charter.pdf. Published April 1, 2018. Accessed May 14, 2019.
- Robinson CL, Bernstein H, Romero JR, Szilagyi P. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger — United States, 2019. MMWR Morb Mortal Wkly Rep. 2019;68(5):112-114. doi: 10.15585/mmwr.mm6805a4.
- Recommended adult immunization schedule for ages 19 years or older, United States, 2019. CDC website. cdc.gov/vaccines/schedules/hcp/imz/adult.html. Reviewed February 5, 2019. Accessed May 14, 2019.
- Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2019. CDC website. cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Reviewed February 5, 2019. Accessed May 14, 2019.
- Kim DK, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older — United States, 2019. MMWR Morb Mortal Wkly Rep. 2019;68(5):115-118. doi: 10.15585/mmwr.mm6805a5.
- Immunization schedule changes. CDC website. cdc.gov/vaccines/schedules/hcp/schedule-changes.html#child. Reviewed February 5, 2019. Accessed May 14, 2019.
https://ift.tt/2ZnpcUH

Comments
Post a Comment